Повернутися до бібліотеки Ромера
Beyond Immuno-Based Allergen Testing

Most commercially available kits for food allergen testing rely on the application of immuno-based methods such as ELISA or lateral flow devices (strip tests). To carry out ELISA, trained personnel are required but numerous samples can be analyzed in parallel by using 48-well or 96-well microtiter plates. In general, the analysis can take between 30 minutes and a few hours.
At present, ELISA is the most widely applied method for the detection and quantification of food allergens. However, although many samples can be analyzed at the same time, these samples can only be tested for one analyte.
Limitations to consider
Due to the high specificity of antibodies towards only one particular allergenic protein and technology related limitations, a separate kit has to be used for each allergen. Furthermore, the high degree of specificity to one allergen might lead to false negative results.
Food processing steps like heat treatment, the addition of acidic compounds or fermentation can modify the target protein structure. These modified allergens can lose their immunological properties and the antibody – target protein complex cannot be formed anymore. This leads to false negative results or reduced quantifications. Strip tests are inexpensive, very easy to use, do not require laboratory equipment, and give results usually in a few minutes. However, most strip tests are only qualitative and rely on antibodies as recognition elements. Therefore, they suffer from the same problems as ELISA tests with highly processed food.
In recent years, alternative analysis methods have been established to overcome at least some of the restrictions of immuno-based tests systems.
Detecting allergens with DNA
PCR (polymerase chain reaction) is a relatively fast and inexpensive method for identifying DNA. This technology, developed in the 1980s, has improved continuously since then. PCR has been used for many years in the fields of medical diagnostics, forensics, environmental monitoring, and the quantification of genetically modified organisms in food and feed. In the early 2000s PCR was applied for the first time to identify the DNA of common food allergens like hazelnut and peanut. Until now, PCR assays for most of the US “big eight” and the 14 EU food allergens have been published.
PCR amplifies small fragments of a target DNA until a sufficient number of copies are obtained for visualization or quantification. By multiplying the analytical target by a factor of 107 to 109, the few molecules of allergen DNA obtained might just be sufficient for the successful detection of allergenic ingredients. Initially developed as a qualitative method, PCR was later modified to become a tool for quantitative analysis by the application of different fluorescent generating dyes or probes. The fact that PCR detects the extremely stable DNA molecule might be an advantage when analyzing highly processed food. DNA tends to be unaffected even by extreme conditions and can therefore still be detected even when most of the proteins have already been degraded or modified in some way. Furthermore, PCR can be used for allergens like celery which cannot be detected by antibodies. Celery has to be labeled in the EU but until now, all attempts to produce reliable antibodies have failed due to the close relationship between celery and other plants like parsley, carrot, coriander or fennel.
Over the last decade, newer DNA detection techniques have been developed. All these so-called isothermal amplification methods are in some way related to the conventional PCR but can be performed almost without any instrumentation.
A simple heating block is used to amplify the target DNA and the subsequent visual detection is realized via fluorescent dyes. Isothermal amplification is usually faster than PCR and less prone to any co-isolated impurities and in many cases even more sensitive.
Опубліковано:
Food Allergens

Why DNA techniques might fail
Although the detection of the DNA of allergenic food compounds might have some advantages over immuno- based methods, this approach suffers from some severe drawbacks. As DNA is the analyte of choice for PCR, it is difficult/ impossible to discriminate between egg or milk and the corresponding tissue DNA of chicken or cow as share identical DNA.
Some samples like egg white or milk only contain minor amounts of DNA but a lot of allergenic proteins and therefore, this method is not suitable for analyzing such types of samples.
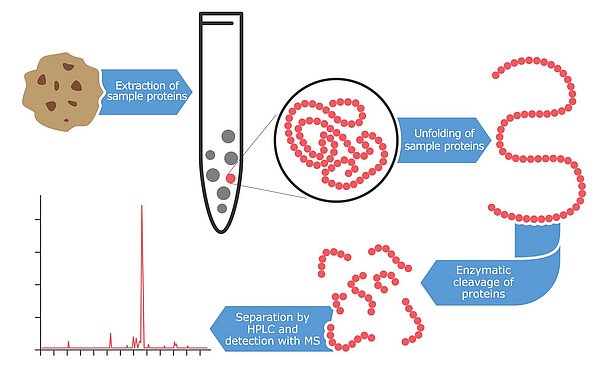
Mass spectrometry: a high-end technology
An even newer technology for detecting and quantifying allergens is mass spectrometry, a high-tech method that identifies proteins and peptides with a very high level of accuracy. The first attempts at applying this technology to allergen detection began in the late 1990s but most of the results were only published in the last few years. The main benefit of using this technology for allergen testing is the high level of confidence and reliability. The instruments have the capability of detecting multiple peptides per protein. Ideally, two to three fragment peptides are analyzed per allergen. The advantage of this approach is that even if proteins are partially degraded or modified due to harsh food processing conditions, the probability of finding at least one intact fragment is quite high. These marker peptides are selected from databases or from literature and must be highly specific for the allergens to be quantified. Furthermore, they are chosen to be resistant to food processing alterations.
This multi-peptide recognition strategy of the allergen is not possible with immuno-based assays. Antibodies usually bind to only one particular (immunogenic) fragment of the allergen. If this small fragment is modified, the recognition of the target might be hampered.
Additionally, mass spectrometry is able to measure several allergens in parallel. These multi-analyte methods have become particularly popular in recent years. This innovative strategy allows a single extraction of a sample to be screened for numerous allergens in a single analysis run. The extraction procedure for mass spectrometry analysis is more laborious than for other approaches. First, the sample is mixed with an extraction buffer often containing dithiothreitol or urea to create a reducing environment that breaks up disulfide bonds of proteins. The remaining sample residue is then removed by centrifugation and the linearized proteins are cleaved with digestion enzymes. Some hours or an overnight duration is required to cut the allergenic proteins with these enzymes into small peptide fragments. Although the preparative steps are time consuming, the resulting peptide solution can then be analyzed for several allergens in parallel using the mass spectrometer. Current multi-methods can quantify up to seven allergens in parallel, but it can be expected that this number might increase dramatically over the next few years. Mycotoxin multi-analyte methods started with a few analytes only some 10 years ago and today, the most advanced assay designs are capable of analyzing more than 400 toxins in parallel.
The best method?
Mass spectrometry can probably be considered the allergen testing method having the most potential for future improvements due to its outstanding reliability, sensitivity and the potential to perform multi-allergen analysis.
However, there is no approach without drawbacks. Mass spectrometry needs highly skilled personnel and the initial investment costs are high due to the expensive instrumentation. Furthermore, the time to result will always be much longer than for immuno methods.
No one-size-fits-all
The perfect method, a gold standard for allergen quantification, does not exist. ELISA and LFDs are the method of choice for the majority of industrial applications. Results can be obtained relatively quickly, costs are moderate to low and personnel can be easily trained to use these tests. For some problems like highly processed testing material or specific analytes, PCR might lead to better results. Mass spectrometry is situated at the upper end of available technologies but is still in its infancy for allergen testing. However, it has, in recent years, become the method of choice for many other analytical challenges. It can be expected that this technology might experience a boost in the field of allergen analysis in near future.