Повернутися до бібліотеки Ромера
Impedance Flow Cytometry and Its Use in Monitoring Food Processing Environments
What is flow cytometry?
Flow cytometry refers to a group of techniques that use a laser or electrical field to count cells suspended in a fluid and to determine some of their physical or chemical properties. Optimally, only one cell at a time flows through the microfluidic channel of the cytometer, which detects variations in the wavelength of light or in electrical charge as each cell or other particles pass through. As flow cytometry generally requires large and expensive devices as well as fastidious preparatory steps, the method has traditionally been limited to laboratory use in fields of application such as research and medicine.
Опубліковано:
Microbiology
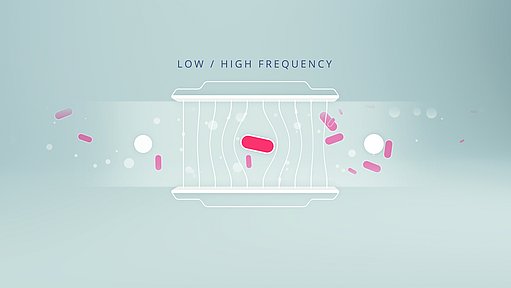
Deploying impedance flow cytometry to count both cells and residue particles
Impedance flow cytometry is derived from the technology underpinning Coulter particle counters, which can size and count particles suspended in electrolytes based on changes in impedance caused by the displacement of electrolytes by the particles. By measuring multiple frequencies at the same time for each passing particle, impedance flow cytometry can discriminate between particles based not only on size, but also electrical properties. This is a powerful variant of flow cytometry as it is very robust and can be used to assess cellular characteristics that are otherwise impossible to measure without the use of molecular tags, such as cell membrane integrity. Therefore, instead of a laser, an impedance flow cytometer employs an alternating current, the varying frequencies of which enable the device to detect, measure the size of and separately count membrane-intact cells and other particles. Compared to other flow cytometric devices, impedance flow cytometers can be light, portable and battery-powered, enabling them to be used where the sample is taken.

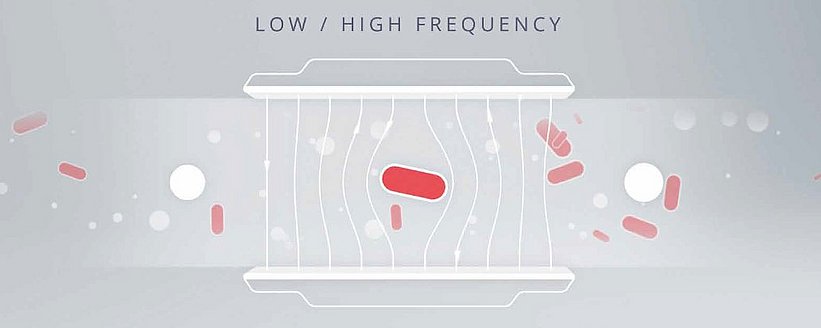
How do impedance flow cytometers tell the difference between cells and other particles?
Impedance flow cytometry takes advantage of the unique electro-magnetic properties of the cell membrane and cytoplasm to distinguish bacteria from other particles. A cell’s membrane and cytoplasm influence the electrical field in a way that is different from other particles in the sample. An example using metallic (conductive) particles, non-conductive particles and intact cells can illustrate this principle most clearly. Regardless of the frequency of the electric field, the conductivity of metallic particles will permit the electrical field to pass through unimpeded. Contrariwise, non-conductive particles such as polystyrene will resist the electrical field; the current will only advance in the liquid medium, which leads to a measurable volume displacement correlating with the particles in the flow channel. Intact cells, however, are unique in that they resemble both non-conductive and metallic particles, depending on the frequency of the electric field. At low frequencies, the isolating quality of a cell’s membrane prevents the electric field from penetrating it, leading to the same kind of displacement as with non-conductive particles. Higher frequencies, however, can partially penetrate the membrane; as such, cells are similar in conductivity to metallic particles. The microelectrodes in impedance flow cytometers generate fields at both low and high frequencies, allowing the device to detect these changes in conductivity and resistance and attribute them in precise numbers to either intact cells or other particles. The detector identifies the target as a bacterium based on the varying degree of impedance or conductivity at these frequencies. The user then receives separate counts of intact cells and other particles.
How does impedance flow cytometry compare to cultural methods?
Cultural methods, the use of agar plates in particular, are the traditional approach to monitoring the sanitation of food processing environments. Yet cultural methods, though well established, have several disadvantages concerning their speed and scope. Cultural methods are slow, requiring between one and ten days for bacteria to grow into countable colonies. These methods measure only what is culturable under the specific conditions of a given test run; a species or other grouping of bacteria may require a specific agar or liquid medium at an exact temperature, degree of light, or humidity, to name just a few variables. Cultural methods also can make no claims to comprehensive measurement of all bacteria in a sample. The “great plate count anomaly,” a conundrum well-known in microbiology, observes that only a small fraction of bacteria in a habitat can be retrieved by culturing. Bacteria in a viable but non-culturable (VBNC) state are alive, but due to stress, idiosyncrasies or less than optimal environmental factors cannot grow on agar or in liquid media. In some cases, they can be cultured after resuscitation, a process that, again, is time-consuming. Some pathogenic bacteria, such as E. coli O157, have been known to enter a VBNC state only to proliferate in later stages down the food chain or in human hosts after ingestion.Furthermore, anaerobic and microaerophilic bacteria require the absence of oxygen or levels of oxygen lower than that of normal atmospheric conditions, respectively. The bacteria in these groups that are culturable require special incubation conditions, adding to the cost of analytical tests. Impedance flow cytometers count all bacteria that pass through the flow channel, regardless of their state (culturable, VBNC, non-culturable, dormant) or growth requirements. Such direct, immediate quantification broadens the scope of a hygiene control program; bacteria that do not multiply until coming into contact with food or potential hosts can also be targeted with impedance flow cytometry. It also allows for taking immediate action whenever cleaning and disinfection does not go according to plan.